| |
Achilles Tendonitis
Prior
to any Sonocur treatment, care must be taken to be certain that the
patient meets the treatment criteria, has no treatment contraindications,
has had the necessary pre-treatment imaging studies, and has read and
signed the proper informed consent.
 |
|
click
to enlarge
|
|
 |
|
click
to enlarge
|
|
PROCEDURE
TECHNIQUE
- Place patient on
treatment table in prone position with a pillow under the head. If the
patient has neck or back discomfort in the prone position, then a pillow
may be placed under the abdomen or thorax
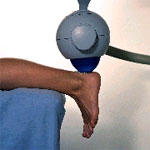
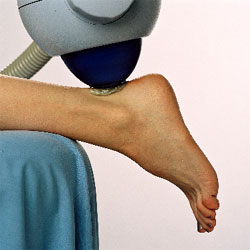
- Position the foot
over the end of the table with the toes pointing to the floor. A pillow
should be placed under the lower leg to slightly flex the knees.
- Identify trigger
point (sore and/or indurated area along the Achilles tendon) by palpation
and mark with pen if necessary.
- Apply transmission
gel over trigger point.
- Swing Sonocur
shock head into place and position over identified trigger point.
- Apply shock waves
initially at lowest energy level (level 1) at 4Hz.
- Move shock head
in small increments until patient reports maximal reproduction of discomfort.
- Accomplish fine
adjustment of shock wave penetration depth by adjusting amount of fluid
in bellows, again with patient feedback to identify maximal trigger
point stimulation.
- Great care must
be taken to precisely identify the exact area of pain. It may require
the use of several shocks (eg.100) to identify this site.
- Depending upon
patient tolerance, gradually increase energy levels to the highest level
that can be maximally tolerated. In Achilles tendonitis this may be
quite variable. For patients with insertional Achilles tendonitis,
only lower energy levels are usually tolerated (levels 1-3). In patients
with nodular tendonitis, with well defined areas of induration
or nodules, medium energy levels (levels 3-5) are usually well tolerated.
Be certain that the patient does not become too uncomfortable. It is
more important to deliver all the shock waves to the correct area at
a lower energy level than miss the mark with higher levels because the
patient is too uncomfortable. AT NO TIME SHOULD AN ENERGY LEVEL GREATER
THAN 5 BE USED IN ANY TYPE OF ACHILLES TENDONITIS.
- Readjust the shock
head position after every 200-400 shocks to precisely treat the maximal
area of tenderness.
- Deliver a total
of 2000 shocks to the affected site after it has been identified.
- Remove shock head
from the treated area and observe the site of application.
- Wipe away the
gel.
- Supply patient
with post treatment instructions and return appointment for additional
treatment or physician visit.
HELPFUL HINTS
- If the patient
is exquisitely tender or has significant discomfort, it may be helpful
to initiate treatment slightly away from the point of maximal tenderness.
After 100-200 shocks, slowly readjust the shock head so that the shock
waves are focused more progressively toward area of maximal tenderness.
Slowly advance to the maximal trigger point and complete the treatment.
- In patients with
nodular Achilles tendonitis, about 30 % will have two (2) nodular tender
trigger areas and approximately 15% will have three (3) areas. In these
cases, each area should be treated with 2000 shock waves but no more
than 6000 shocks delivered in any case.
- Some patients
may experience shock wave exit sensations opposite the application site
at medium or higher energy settings. This is normal and causes no harm.
- Inform the patient
that it is usual to have soreness after treatment and that often the
pain will be worse for a few days until healing begins. Explain that
if multiple treatments are necessary, that subsequent treatments can
sometimes be more uncomfortable than the initial treatment. In addition,
emphasize that healing may take several weeks to occur and that one
should not expect maximal improvement until 12 weeks after the last
treatment.
Sonorex Sonocur
Treatment Protocols are copyright protected.
© 2012 Sonorex. All rights reserved.
The Sonocur® Orthopedic Extracorporeal Shockwave system is available in Canada and other countries where regulatory approval has been obtained. The Sonocur® Basic is FDA approved in the United States for the treatment of chronic lateral epicondylitis (tennis elbow).
© 2012 Sonorex. All rights reserved. By using this service, you accept the terms of our Visitor Agreement. Please read it. The material sonorex.ca is for informational purposes only and is not a substitute for medical advice or treatment for any medical conditions. You should promptly seek professional medical care if you have any concern about your health, and you should always consult your physician before starting a fitness regimen.
|
 |
|
click
to enlarge
|
 |
|
click
to enlarge
|
|